Muscle Mania Part III: Mechanisms For More Muscle
Diving into the molecular pathways for increasing muscle protein synthesis.
Thus far in the Muscle Mania Series (I, II), we have explored the structure and mechanistic function of muscle tissue, as well as the pivotal processes that dictate muscle size – muscle protein synthesis and breakdown. During this journey, we have established that muscular hypertrophy centers around building and incorporating myofilaments into new sarcomeres, a concept which we can understand through the analogy of improving and adding to a house, where the house represents a muscle fiber. Today, we will expand our picture of muscle hypertrophy by focusing on the pathways and mechanisms through which our muscle cells initiate and propagate construction: ramping up muscle protein gene expression by activating transcription factors, increasing nuclear capacity via satellite cell addition, and expanding translational capacity through ribosome biogenesis. Before we get started, check out the recap below to refresh yourself on what we’ve covered so far.
Due to email length restrictions, I could not fit my reference list on this post, but you can find it here.
Recap
Muscles are ultimately composed of contractile proteins, called myofilaments, which are organized into structural units, called sarcomeres.
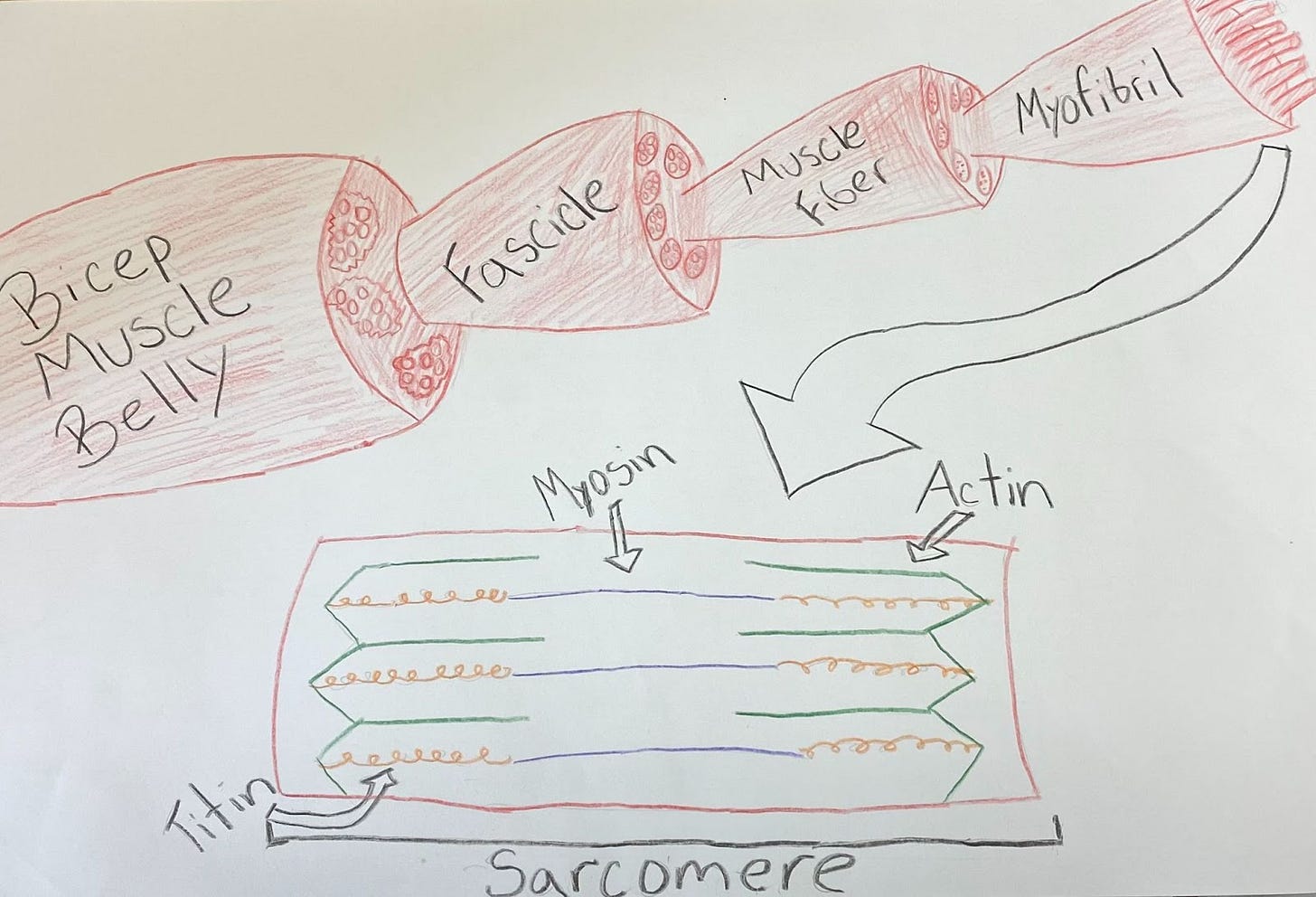
During a muscular contraction, myosin filament heads bind to and pull on surrounding actin filaments, sliding the filaments past each other – similar to how a person (the myosin filament) uses their arms (the myosin heads) as levers to pull on and slide across the ground (the actin filaments) during a low crawl. This motion moves the ends of the sarcomere towards the sarcomere’s center, which shortens the entire structure.
Muscular hypertrophy, or muscular growth, involves building and incorporating myofilaments into new sarcomeres to grow the overlying muscle.
Muscle hypertrophy and atrophy are the results of an interplay between muscle protein synthesis (MPS) and muscle protein breakdown (MPB); interestingly, both processes are essential for building muscle.
Muscle hypertrophy is analogous to making improvements and additions to a house, in that adding new bricks, planks, and shingles – analogous to MPS – is the central mechanism for making the house thicker or taller, and removing and replacing old, decrepit, or damaged portions of the house – analogous to MPB – serves a supporting role.
Protein synthesis involves a macro-level signal (like lifting weights or eating protein) inducing a cellular signal at the micro-level, which provokes transcription of specific parts of the cell’s DNA (i.e. specific genes) into mRNA, the translation of that mRNA by ribosomes, and construction of the correlating proteins. During muscle protein synthesis, those genes code for specific muscle proteins that will be incorporated into sarcomeres.
Cell Signaling and Amplification
Prior to diving into the meat and potatoes below, let’s briefly talk about cell signaling; specifically, if we are to understand how things like lifting weights induce MPS, we should understand the concepts of signaling cascades and amplification. Imagine you are a middle-schooler in social studies class back in the dark ages – the early 2000’s – and you want to ask your friend, Johnny, to come over and go swimming after school. You do have a cell phone, but it’s one of those prehistoric devices with which it takes roughly 17-19 seconds to type “Hey,” because you have to make 2 clicks just to get to the, “H,” after which you have to wait for the letter to stop blinking before you can move on to the next one. For this reason, instead of sending a text, it’s much easier to write Johnny a note and pass it across class. This process, in which your original message goes to Sally, then David, Jimmy, Michelle, and Margaret before finally reaching Johnny is similar to the cell signaling cascades that occur in all of your cells, where the initial message might come in the form of a chemical interaction – like a molecule binding to a receptor on the cell – or a mechanical force – like fluid build up or tension – acting on the cell. Now, imagine that, instead of the note making it all the way to Johnny, each person in the message chain has a unique way of passing on the signal. For example, after you pass the note to Sally, she draws a picture of a swimming pool with a question mark and passes that to David. Next, David sees the picture and uses sign language to send the message to Jimmy who communicates the signal to Michelle by tapping on his desk in Morse Code. This pattern of varying communication modalities represents how cell signaling cascades consist of a host of different signaling methodologies – some of which involve adding a chemical group to the next molecule in the chain, while others involve removing a chemical group from the next molecule in the chain — all of which ultimately lead to a change in gene expression and/or enzyme activity within the cell.

Taking things a step further, amplification is the process through which your cells magnify that initial message into many chains of cellular communication. It is easy to view amplification through the analogy of a packed bar. It all starts with Morgan accidentally bumping into and spilling her drink on Jarred who then bumps into and spills his drink on both Tyler and Emma. This leads to a cascade of events, whereby Tyler and Emma bump into two more people each, and those people bump into another two people each, and so on and so forth. All of a sudden, Morgan’s single interaction with Jarred has created countless chains of interactions involving hundreds of people in the bar. Similarly, cell signaling cascades often involve one molecule activating multiple of their target molecules which then activate multiple of their target molecules, and so on. In this way, activating one cell surface receptor or mechanical sensor can produce a large response within the cell.

Transcription Factors
As we have already established, increasing MPS is the backbone of muscular hypertrophy. Researchers are still ironing out the exact pathways through which resistance training and diet induce the molecular processes involved in muscle growth; however, some of the main culprits include mechanotransducers, like integrin adhesion complexes and dystrophin glycoprotein complexes, as well as chemical signaling molecules, such as insulin-like growth factor 1 (IGF-1), androgens (like testosterone), insulin, and amino acids. (I, II, III, IV, V, VI, XII) Once stimulated, these sensors and receptors trigger signaling cascades within the cell that ultimately lead to alterations in MPS and MPB by activating transcription factors associated with muscle protein genes.
If we return to our construction analogy from the last post, transcription factors are similar to the ID badges or keys that workers would require in the shed (nucleus) to begin copying the instructions (DNA) for building parts of the house (proteins). Once activated, transcription factors act like gatekeepers, assembling upon specific locations on the cell’s DNA and initiating transcription of the genes at those locations by enabling the enzymes that conduct the actual copying of the DNA and production of the mRNAs. Transcription factors associated with muscular hypertrophy include myocyte enhancer factor-2 (MEF2), serum response factor (SRF), upstream binding factor (UBF), paired box-7 (Pax7), Yes-associated protein 1 (Yap)and SMAD2/3. (I, II, V, VI, VII, VIII, XII)
Although research around the pathways that prompt hypertrophy-related transcription factors is ongoing, the protein kinase B/phosphatidylinositol-3 kinase (Akt/PI3K), mammalian target of rapomycin complex 1 (mTORC1), mitogen-activated protein kinases (MAPKs), Myostatin (MSTN), and calcium-related pathways are viewed as key players. (I, II, III, IV, V, VI, VII, VIII, IX, XII) In essence, the goal of weight lifting and dieting for muscle gain is to activate these pathways that, in turn, activate hypertrophy-related transcription factors and induce the desired changes in MPS and MPB that will drive increased production and incorporation of myofilaments in myofibers.
Check out this review by Schiaffino Et al. for a deeper dive into the molecular signaling pathways behind hypertrophy.
Satellite Cells
Ultimately, there is only so much transcription that can occur within a single nucleus; consequently, researchers believe that increasing the number of nuclei within myofibers is a key mechanism for increasing MPS and furthering hypertrophy. In terms of our construction analogy, this would be like adding more sheds around the property, increasing the workers’ instruction copying capacity. With more workers (enzymes) creating more instruction copies (mRNAs) the crew can build more structures for the house (MPS) during a given period of time.
Researchers believe that satellite cells, stem cells residing in muscle tissue, drive nuclei accumulation in myofibers by fusing with and donating their nuclei to said myofibers.(I, II, IV, VI, VII, X, XI, XII) As stem cells, satellite cells possess self-renewal abilities, meaning that they can divide indefinitely once activated, theoretically offering an endless pool of nuclei for growing myofibers. Satellite cell involvement in muscle hypertrophy is supported by several lines of evidence: extreme responders to resistance training have greater amounts of satellite cells per muscle fiber at baseline (X) as well as greater increases in both satellite cells and myonuclei (nuclei within myofibers) per muscle fiber compared to non-responders following resistance training (X, XI) ; genetic knockout of satellite cell related genes in mice impairs hypertrophy (I); satellite cell number increases acutely in myofibers in the days following resistance training as well following multiple weeks of resistance training (II, VI).
With that said, some research suggests that satellite cell contribution is not necessary for hypertrophy, such as the mice studies that do show hypertrophy despite impaired satellite cell function. (II, V) For this reason, some researchers have proposed that satellite cell contribution is only necessary once a myofiber has hypertrophied to the point where its myonuclei capacity is not sufficient for further hypertrophy – a concept referred to as the ceiling effect. Interestingly, one group of researchers hypothesized that an individual’s capacity to ramp up transcription and translation – particularly their capacity for ribosome biogenesis –- could dictate their need for satellite cell fusion and increased myonuclei. (V)
Check out Bazgir Et al.’s review for more on how satellite cells impact hypertrophy.
Ribosome Biogenesis
The third and final mechanism through which we will escalate our MPS potential revolves around our translational capacity. In our prior discussion, we related ribosomes to machines at a construction site that build structures (proteins) for incorporation into the home (the myofiber). So far, we have discussed how to cap off the amount of mRNA available for ribosomes to translate by ramping up transcriptional capacity; however, we can also expand our translational capacity by growing more ribosomes in our muscle cells, a process referred to as ribosome biogenesis. The data supporting this ribosome biogenesis hypothesis are multiple, demonstrating acute and long-term increases in ribosomal RNA (an indicator of ribosome production) following resistance training (I, II, V, VIII), correlations between measures of ribosome biogenesis and degree of hypertrophy following resistance training (I, II, V, VIII), and mitigated hypertrophy response in mice following inhibition of enzymes involved in ribosome biogenesis (XI). In addition to increasing the number of ribosomes, there is evidence that existing ribosomes improve their ability to synthesize proteins, a concept referred to as translational efficiency, during hypertrophy. (II, VIII)
So, if we want to initiate MPS, we activate transcription factors that correspond to muscle protein genes, and we can magnify that transcription by increasing the number of nuclei within our myofibers through the help of satellite cells. Finally, once we’ve jacked up transcription, we can tweak things further by improving translational capacity and efficiency through ribosome biogenesis.
For a deeper dive into ribosome biogenesis, check out this review by Brook Et al.
Well, now that we’ve covered the nerdy, cell biology mechanisms pulling the strings behind the scenes of hypertrophy, we can get to the more functional questions everybody cares about, like, “How do exercise and diet impact muscular hypertrophy?”



